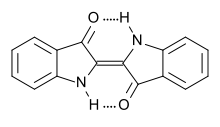
- Colorante índigo
-
El colorante índigo es una tintura con un distintivo color azul (ver índigo). El compuesto químico constituyente del índigo es la indigotina.
Los antiguos extraían el colorante natural de varias especies de plantas, como también de especies zoológicas como el famoso caracol Hexaplex trunculus, pero actualmente casi todo se produce por síntesis química.
Además de otros usos, se industrializa para producir la tela denim para jeans azules. La forma de índigo usada en alimentación es llamada "indigotina", y está listada en EE. UU. como FD&C Blue Nº 2, y en la Unión Europea como Número E: E132.
Contenido
Fuentes y usos
Una variedad de plantas, incluyendo a Isatis tinctoria, han provisto de índigo a través de la historia, aunque la mayoría del índigo natural se obtiene de spp. del género Indigofera, nativo de los trópicos. En climas templados el índigo puede obtenerse de Isatis tinctoria y de Polygonum tinctorum, aunque las spp. de Indigofera rinden más tintura. La especie comercial de índigo en Asia fue el índigo verdadero Indigofera tinctoria, o llamado Indigofera sumatrana). En Centro y Sudamérica las dos especies Indigofera suffruticosa (añil) y Indigofera arrecta (índigo de Natal) fueron los más importantes.
El "índigo natural" fue la única fuente del tinte hasta 1900. Dentro de un corto tiempo, sin embargo, el índigo sintético desplazó completamente al natural, y hoy casi todo se produce vía síntesis.
En EE.UU., el uso primario de uso para el índigo, es como un tinte para ropa de trabajo de algodón y blue jeans. Más de 1.000 millones de pares de jeans en todo el mundo se tiñen con índigo blue. Por muchos años, el índigo fue usado para dar el "azul naval profundo" e lana.
El índigo no se une fuertemente a la fibra, y los lavados repetitivos pueden lentamente irremoviendo el tinte.
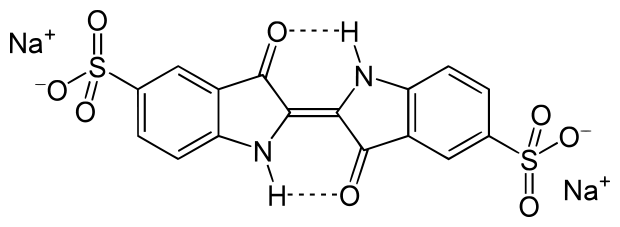
Además, el índigo se usa como colorante de alimento, y está listada en EE.UU. como FD&C Blue Nº 2. El specification FD&C Blue Nº 2 incluye tres sustancias, de las cuales la mejor es la sal sódica de Indigotindisulfonato.
El indigotinesulfonato se usa también como un tinte en pruebas de la función renal, como reactivo en detectar nitratos y cloratos, y en el testeo de leche.
Historia
El índigo es uno de los más antiguos tintes usados en tintura textil y en imprenta. Muchos países asiáticos, como India, China, Japón, han usado el índigo como tintura por centurias. También era conocido en antiguas civilizaciones de Mesopotamia, Egipto, Grecia, Roma, Bretaña prehistórica, Mesoamérica, Perú, Irán, África.
Se cree que India es el más antiguo centro de tintura índigo del Viejo Mundo. Y suministraba índigo a Europa en la era Greco-Romana. La asociación de India con el índigo se refleja en la palabra griega para tintura, que era indikon. Los romanos usaban el término indicum, pasando a la lengua itálica y eventualmente al inglés con indigo.
En la Mesopotamia, una tablilla neobabilónica en escritura cuneiforme del s. VII a. C., da una receta para teñir lana, donde la lana coloreada con lapislázuli (uqnatu) se producía por inmersiones repetidas y aireando la prenda. El índigo probablemente se importada de la India.
Los romanos usaban índigo como pigmento de pintura y para uso medicinal y cosmético. Era un ítem de lujo importado a la región del Mediterráneo desde India por mercaderes árabes.
El índigo siguió siendo un "commodity" raro en Europa a través de la Edad Media. El "woad" o glastum, un tinte químicamente idéntico, derivado de la planta Isatis tinctoria (Brassicaceae), se usaba en su reemplazo.
A fines del s. XV, el explorador portugués Vasco da Gama descubre una ruta marina a la India. Así se establece un mercado directo con el Medio Oriente, las islas de las Especies, China, Japón. Los importadores ahora podían evitar los pesados impuestos y gabelas impuestos por Persia, Levante, Grecia, y las distancias y peligrosidad de las rutas terrestres previamente que habían sido usadas. Consecuentemente, la importación y uso del índigo en Europa creció significativamente. La mayoría del índigo europeo de Asia llegaba a través de puertos en Portugal, Holanda, e Inglaterra. España importaba el tinte de sus colonias en América del Sur. Muchas de las plantaciones del colorante índigo fueron establecidas por poderes europeos en climas tropicales; era un cultivo principal en Jamaica y Carolina del Sur, donde todo o la mayoría del trabajo era realizado por esclavos traídos de África. Las plantaciones de índigo también abundaron en la Islas Vírgenes. Sin embargo, Francia y Alemania prohibieron el índigo importado en los 1500s para proteger la industria de tintes local.
Indigo was the foundation of centuries-old textile traditions throughout West Africa. The use of indigo here pre-dated synthetics. From the Tuareg nomads of the Sahara to Cameroon, clothes dyed with indigo signified wealth. Women dyed the cloth in most areas, with the Yoruba of Nigeria and the Manding of Malí particularly well known for their expertise. Among the Hausa male dyers working at communal dye pits were the basis of the wealth of the ancient city of Kano, and can still be seen plying their trade today at the same pits.
In Japan, indigo became especially important in the Edo period when it was forbidden to use silk, so the Japanese began to import and plant cotton. It was difficult to dye the cotton fiber except with indigo. Many years later the use of indigo is very much appreciated as a color for the summer Kimono Yukata, as the blue sea and the nature are recalled on this traditional clothing.
In 1865 el químico alemán Johann Friedrich Wilhelm Adolf von Baeyer began working with indigo. His work culminated in the first synthesis of indigo in 1880 from o-nitrobenzaldehyde and acetona upon addition of dilute sodium hydroxide, barium hydroxide, or ammonia and the announcement of its chemical structure three years later. BASF developed a commercially feasible manufacturing process that was in use by 1897, and by 1913 natural indigo had been almost entirely replaced by synthetic indigo. In 2002, 17,000 tons of synthetic indigo were produced worldwide.
In the nineteenth century, the British obtained much indigo from India. With the coming of the synthetic substitute, the demand for natural indigo dropped, and for many indigo farmers the raising of indigo became umprofitable.
In literature, the play Nildarpan by Dinabandhu Mitra is based on the indigo slavery and forceful cultivation of indigo in India. It played an essential part in the Bengali indigo revolt of 1858 called Nilbidraha.
Developments in dyeing technology
Indigo is a challenging dye to use because it is not soluble in water; to be dissolved, it must undergo a chemical change. When a submerged fabric is removed from the dyebath, the indigo quickly combines with oxygen in the air and reverts to its insoluble form. When it first became widely available in Europe in the sixteenth century, European dyers and printers struggled with indigo because of this distinctive property. It was also a toxic substance that, by requiring several chemical manipulations, had many opportunities to injure workers. In fact, during the 19th century, English poet William Wordsworth referred to the plight of indigo dye workers of his hometown of Cockermouth in his autobiographical poem "The Prelude". Speaking of their dire working conditions and the empathy that he feels for them, he writes, "Doubtless, I should have then made common cause/ With some who perished; haply perished too,/ A poor mistaken and bewildered offering - / Unknown to those bare souls of miller blue."
A preindustrial process for dyeing with indigo, used in Europe, was to dissolve the indigo in stale urine. Urine reduces the water-insoluble indigo to a soluble substance known as indigo white or leucoindigo, which produces a yellow-green solution. Fabric dyed in the solution turns blue after the indigo white oxidizes and returns to indigo. Synthetic urea to replace urine became available in the 1800s.
Another preindustrial method, used in Japan, was to dissolve the indigo in a heated vat in which a culture of thermophilic, anaerobic bacteria was maintained. Some species of such bacteria generate hydrogen as a metabolic product, which can convert insoluble indigo into soluble indigo white. Cloth dyed in such a vat was decorated with the techniques of shibori (tie-dye), kasuri, katazome, and tsutsugaki. Examples of clothing and banners dyed with these techniques can be seen in the works of Hokusai and other artists.
Two different methods for the direct application of indigo were developed in England in the eighteenth century and remained in use well into the nineteenth century. The first method, known as pencil blue because it was most often applied by pencil or brush, could be used to achieve dark hues. Arsenic trisulfide and a thickener were added to the indigo vat. The arsenic compound delayed the oxidation of the indigo long enough to paint the dye onto fabrics.
The second method was known as china blue due to its resemblance to Chinese blue-and-white porcelain. Instead of using an indigo solution directly, the process involved printing the insoluble form of indigo onto the fabric. The indigo was then reduced in a sequence of baths of iron(II) sulfate, with air-oxidation between each immersion. The china blue process could make sharp designs, but it could not produce the dark hues possible with the pencil blue method.
Around 1880 the glucose process was developed. It finally enabled the direct printing of indigo onto fabric and could produce inexpensive dark indigo prints unattainable with the china blue method.
Chemical properties
Indigo is a dark blue crystalline powder that melts at 390°–392 °C. It is insoluble in water, alcohol, or ether but soluble in chloroform, nitrobenzene, or concentrated sulfuric acid. The chemical structure of indigo corresponds to the formula C16H10N2O2.
The naturally occurring substance is indican, which is colorless and soluble in water. Indican can easily be hydrolyzed to glucose and indoxyl. [[]], such as by exposure to air, converts indoxyl to indigo.
The manufacturing process developed in the late 1800s is still in use throughout the world. In this process, indoxyl is synthesized by the fusion of sodium phenylglycinate in a mixture of sodium hydroxide and sodamide.
Several simpler compounds can be produced by decomposing indigo; these compounds include aniline and picric acid. The only chemical reaction of practical importance is its reduction by urea to indigo white. The indigo white is reoxidized to indigo after it has been applied to the fabric.
Indigo treated with sulfuric acid produces a blue-green color. It became available in the mid-1700s. Sulfonated indigo is also referred to as Saxon blue or indigo carmine.
Tyrian purple was a valuable purple dye in antiquity. It was made from excretions of a common Mediterranean Sea snail. In 1909 its structure was shown to be 6,6′-dibromoindigo. It has never been produced synthetically on a commercial basis.
The SMILES structure of indigo is Plantilla:SMILES and its CAS number is Plantilla:CASREF.
Chemical synthesis
Indigo may be synthetically manufactured in a number of different ways. The original method, first used to synthesise indigo by Heumann in 1897, involves heating N-(2-Carboxyphenyl)glycine acid to 200 °C in an inert atmosphere with sodium hydroxide. This produces indoxyl-2-carboxylic acid, a material that readily decarboxylates and oxidises in air to form indigo.
 Heumann's original synthesis of Indigo
Heumann's original synthesis of IndigoThe modern synthesis of indigo is slightly different from that route originally used and its discovery is credited to Pfleger in 1901. In this process, N-phenylglycine is treated with an alkaline melt of sodium and potassium hydroxides containing sodamide. This produces indoxyl, which is subsequently oxidised in air to form indigo.
 Pfleger's modern synthesis of Indigo
Pfleger's modern synthesis of IndigoEnlaces externos
- Plant Cultures: botany, history and uses of indigo
- Pubchem page for indigotine
- FD&C regulation on indigotine
Bibliografía
- Jenny Balfour-Paul, Indigo (London, British Museum 1998).
- Ferreira E. S. B., Hulme A. N., McNab H., Quye A. (2004). «The natural constituents of historical textile dyes». Chemical Society reviews 33 (6). doi:.
Categorías:- Pigmentos
- Colorantes
- Compuestos heterocíclicos
Wikimedia foundation. 2010.